Cellular stress mechanism links microglia to Alzheimer’s disease progression
New research from CUNY identifies how brain immune cells drive neurodegeneration through stress-induced toxic lipid production, offering potential therapeutic targets.
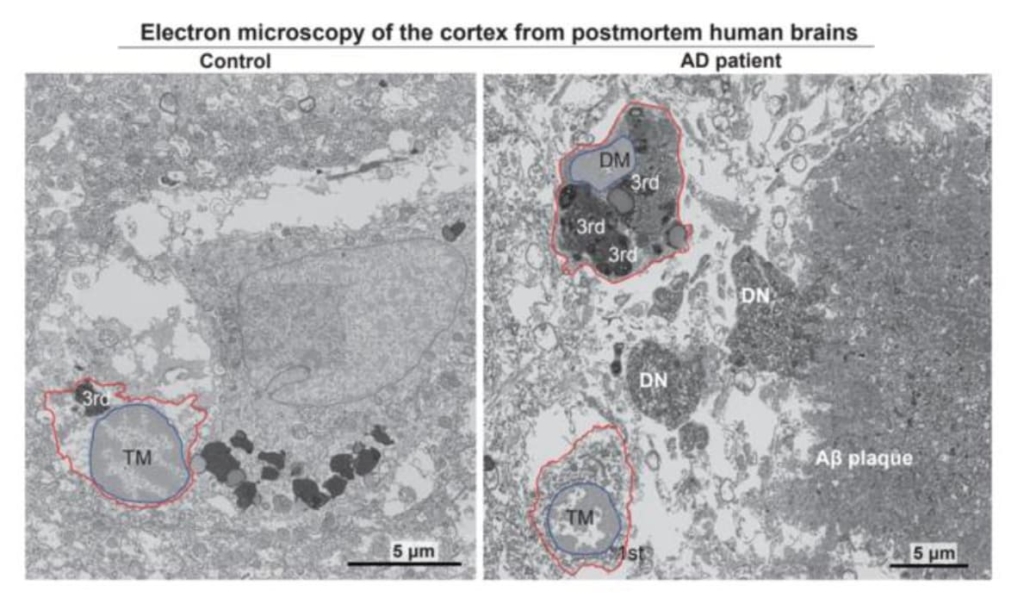
Electron micrographs show typical microglia in the prefrontal cortex of a 92-year-old healthy female (left) and dark microglia a 91-year-old female patient with Alzheimer’s disease (right). © Anna Flury
Scientists at the Advanced Science Research Center at CUNY Graduate Center have uncovered a critical cellular mechanism that helps explain how brain immune cells contribute to the progression of Alzheimer’s disease. The research, published in the journal Neuron on 23 December 2024, reveals how cellular stress in microglia leads to the production of toxic lipids that damage neurons and other essential brain cells.
Understanding microglial behaviour
The study focuses on microglia, the brain’s primary immune cells, which have emerged as key players in Alzheimer’s disease pathology. These cells exhibit a dual nature in the disease process, with some populations protecting brain health whilst others contribute to neurodegeneration. The research team, led by Professor Pinar Ayata, identified a specific subset of microglia known as “dark microglia” that accumulate in the brains of Alzheimer’s patients at twice the levels found in healthy aged individuals.
Stress pathway discovery
The researchers identified that the integrated stress response (ISR) pathway triggers these dark microglia to synthesise and release toxic lipids. These harmful compounds subsequently damage neurons and oligodendrocyte progenitor cells, both of which are crucial for normal brain function and particularly affected in Alzheimer’s disease.
“We set out to answer what are the harmful microglia in Alzheimer’s disease and how can we therapeutically target them,” explained Professor Ayata. “We pinpointed a novel neurodegenerative microglia phenotype in Alzheimer’s disease characterized by a stress-related signalling pathway.”
Therapeutic implications
The investigation revealed promising therapeutic potential through the targeting of specific cellular mechanisms. In mouse models, the researchers demonstrated that inhibiting either ISR activation or lipid synthesis prevented both synapse loss and the accumulation of neurodegenerative tau proteins, two hallmarks of Alzheimer’s disease.
Co-lead author Anna Flury, a PhD student at the CUNY Graduate Center’s Biology Program, emphasised the significance of their findings: “These findings reveal a critical link between cellular stress and the neurotoxic effects of microglia in Alzheimer’s disease. Targeting this pathway may open up new avenues for treatment by either halting the toxic lipid production or preventing the activation of harmful microglial phenotypes.”
Future directions
The research suggests potential new therapeutic approaches through the development of drugs that target either specific microglial populations or their stress-induced mechanisms. According to co-lead author Leen Aljayousi, such treatments could potentially slow or reverse disease progression, offering hope to millions of affected individuals and their families.
The study represents a significant advance in understanding the cellular mechanisms underlying Alzheimer’s disease and highlights the crucial role of microglial health in maintaining brain function. The findings suggest that targeting the ISR pathway or lipid synthesis in microglia could provide a novel therapeutic strategy for treating this devastating neurodegenerative condition.
Reference:
Flury, A., Aljayousi, L., & Ayata, P. (2024). A neurodegenerative cellular stress response linked to dark microglia and toxic lipid secretion. Neuron. December 23, 2024.

