Groundbreaking technique renders skin transparent, opening new avenues for diagnostic imaging
Researchers have developed a novel method to make skin and underlying tissues transparent using a common food dye, heralding potentially far-reaching applications for a wide range of medical diagnostics and certain treatments.
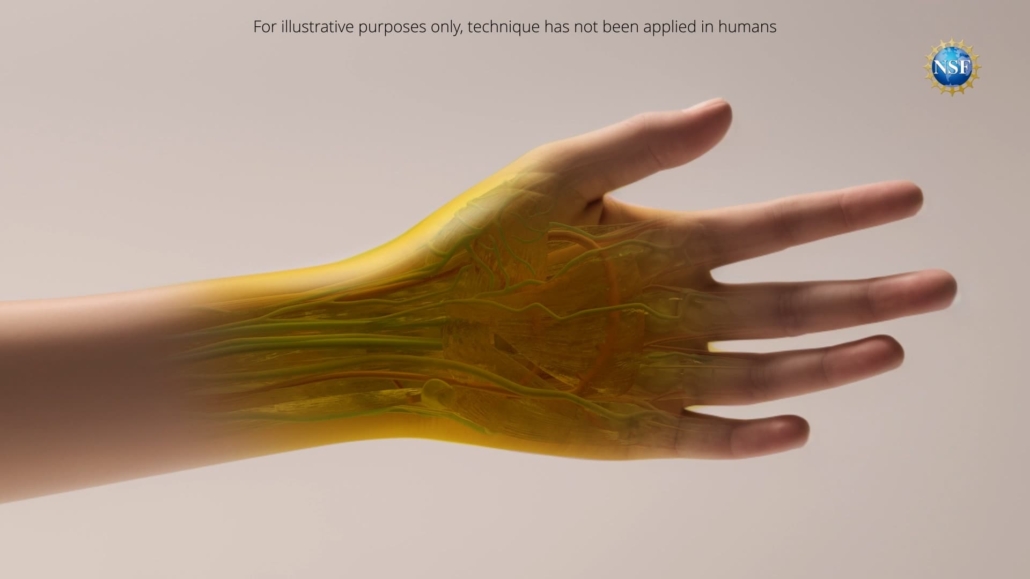
A team of researchers from Stanford University has unveiled a groundbreaking technique that can render skin and underlying tissues transparent, potentially transforming a range of medical diagnostics and some treatments. The study, published in the 6 September 2024 issue of Science [1], demonstrates how a topical application of a common food dye can safely and reversibly make biological tissues transparent to visible light.
The science behind transparency
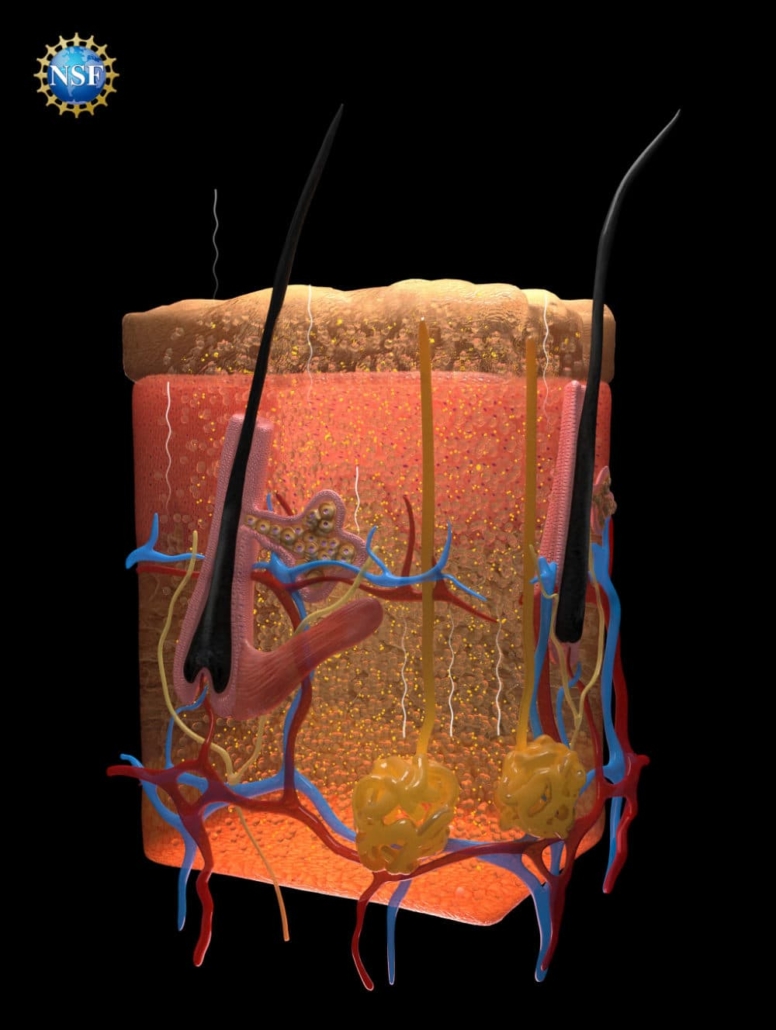
The key to this remarkable achievement lies in the researchers’ deep understanding of how light interacts with biological tissues. Guosong Hong, assistant professor of materials science and engineering at Stanford University and a recipient of the U.S. National Science Foundation CAREER grant, explains that the opacity of biological tissues is primarily due to light scattering.
This scattering occurs because different components within tissues – such as fats, cellular fluids, and proteins – have varying refractive indices. When light passes through these closely packed materials, it bends and scatters, creating the opaque appearance we associate with biological tissues.
To overcome this challenge, the research team developed a method to match these different refractive indices, allowing light to pass through unimpeded. They discovered that certain dyes, particularly those most effective at absorbing light, could also be highly effective at directing light uniformly through a range of refractive indices.
Tartrazine: The key ingredient
After extensive research and experimentation, the team identified tartrazine – commonly known as FD & C Yellow 5 – as a particularly effective dye for this purpose. When dissolved in water and absorbed into tissues, tartrazine molecules are structured in a way that matches refractive indices and prevents light scattering, resulting in transparency.
The researchers first tested their predictions on thin slices of chicken breast, observing that as tartrazine concentrations increased, the refractive index of the fluid within the muscle cells rose until it matched that of the muscle proteins, rendering the slice transparent.
From theory to practice
Building on their success with ex vivo tissues, the team then applied a temporary tartrazine solution to live mice. When applied to the scalp, the skin became transparent, revealing the intricate network of blood vessels crisscrossing the brain. Similarly, when applied to the abdomen, the skin faded within minutes, allowing observation of intestinal contractions and movements caused by heartbeats and breathing.
Remarkably, the technique resolved features at the micron scale and even enhanced microscope observations. The effects were fully reversible, with tissues returning to normal opacity when the dye was rinsed off. Furthermore, any excess dye was safely excreted within 48 hours, with no apparent long-term effects.
Potential applications in medicine
The implications of this research for medicine are far-reaching. Hong suggests that the technology could make veins more visible for blood draws, simplify laser-based tattoo removal, and assist in the early detection and treatment of cancers. “Certain therapies use lasers to eliminate cancerous and precancerous cells, but are limited to areas near the skin’s surface. This technique may be able to improve that light penetration,” he notes.
The researchers also speculate that injecting the dye could lead to even deeper views within organisms, with significant implications for both biology and medicine.
A multidisciplinary approach
The project, which began as an investigation into how microwave radiation interacts with biological tissues, drew on a range of scientific disciplines and resources. The team relied on concepts from optics textbooks from the 1970s and 1980s, including Kramers-Kronig relations and Lorentz oscillation, to predict how dyes could raise the refractive index of biological fluids to match surrounding fats and proteins.
The research also made use of various analytical systems, including a decades-old ellipsometer at the Stanford Nano Shared Facilities, part of the NSF National Nanotechnology Coordinated Infrastructure. This tool, more commonly used in semiconductor manufacturing, proved crucial in predicting the optical properties of the target dyes.
Looking to the future
With methods grounded in fundamental physics, the researchers hope their approach will launch a new field of study matching dyes to biological tissues based on optical properties. This could potentially lead to a wide range of medical applications.
As Hong reflects on the journey, he acknowledges the crucial role of NSF support: “The NSF CAREER award was my first major funding, and it arrived at a particularly challenging time, during the darkest moments of the pandemic. The flexibility and encouragement from the NSF awards were crucial in keeping me on track and allowed me the freedom to explore new and uncharted territories in my field.”
While the technique has not yet been tested on humans, and caution must be exercised with the use of dyes, this research represents a significant step forward in our ability to visualise and understand the inner workings of the body. As further studies are conducted, the medical community eagerly anticipates the potential applications of this groundbreaking technology in improving diagnostics, treatment, and overall patient care.
Please note: The technique described above has not been tested on humans. Dyes may be harmful. Always exercise caution with dyes and do not consume directly, apply to people or animals, or otherwise misuse.
Reference:
Ou, Z., et al. (2024). Achieving optical transparency in live animals with absorbing molecules. Science. doi: https://doi.org/10.1126/science.adm6869