Novel marine-derived compounds show promise in combating tuberculosis
Researchers from German universities have identified a new class of molecules that could pave the way for innovative tuberculosis treatments, offering hope in the face of rising antibiotic resistance.
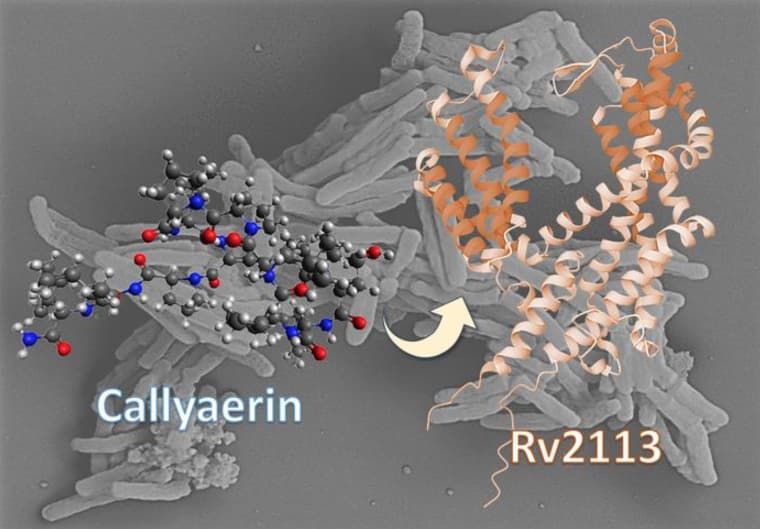
The non-essential membrane protein Rv2113 as a target structure of callyaerin in M. tuberculosis. (Fig.: HHU/Rainer Kalscheuer)
In a groundbreaking study, scientists from Heinrich Heine University Düsseldorf (HHU) and the University of Duisburg-Essen (UDE) have uncovered a novel approach to tackling tuberculosis (TB), one of the world’s deadliest infectious diseases. The research, published in the journal Cell Chemical Biology [1], reveals that a group of molecules known as callyaerins can effectively inhibit the growth of Mycobacterium tuberculosis, the bacterium responsible for TB, through a mechanism entirely distinct from current antibiotic treatments.
A new weapon in the arsenal against tuberculosis
Tuberculosis remains a significant global health threat, with over 10 million new cases reported annually. The World Health Organisation (WHO) estimates that 1.6 million people succumbed to the disease in 2021 alone, underscoring the urgent need for new therapeutic strategies. This urgency is further compounded by the increasing prevalence of drug-resistant strains of M. tuberculosis, which have rendered many existing antibiotics ineffective.
The research team, led by Professor Dr Rainer Kalscheuer from HHU’s Institute of Pharmaceutical Biology and Biotechnology and Professor Dr Markus Kaiser from UDE’s Center of Medical Biotechnology, focused their efforts on callyaerins, a class of cyclopeptides naturally occurring in marine sponges. Through chemical synthesis, the team not only recreated these compounds but also developed more potent derivatives not found in nature.
Dr David Podlesainski from UDE, one of the study’s lead authors, explained the significance of this achievement: “We have succeeded in chemically synthesising the substance that occurs naturally in marine sponges in order to test its effect on tuberculosis bacteria in cell cultures. This has enabled us to produce new, more potent derivatives that do not exist in nature. Such chemical synthesis needs to be successful before a potential active agent can be used as a drug on a large scale.”
Targeting the bacterial Achilles’ heel
The researchers discovered that callyaerins specifically target a membrane protein in M. tuberculosis called Rv2113. While this protein is not essential for the bacterium’s survival, its disruption by callyaerins severely impairs the pathogen’s metabolism and growth. Importantly, the compounds showed no adverse effects on human cells, suggesting a potentially safe therapeutic approach.
Emmanuel Tola Adeniyi, a doctoral researcher at HHU and co-lead author of the study, elaborated on the mechanism: “The callyaerins attack a specific membrane protein of M. tuberculosis called Rv2113, which is not essential for the viability of the bacterium. This comprehensively disrupts the metabolism of the bacterium, hindering its growth. By contrast, human cells remain unaffected by the callyaerins.”
A paradigm shift in antibiotic development
The discovery of callyaerins’ mechanism of action represents a significant departure from traditional antibiotic strategies. Prof. Kalscheuer highlighted this distinction: “With the callyaerins, we have discovered a new mechanism of action. Unlike other antibiotics, these substances do not block vital metabolic pathways in the bacterial cell. Instead, they directly attack a non-essential membrane protein of the bacterium, which has not been considered as a potential target before.”
This novel approach opens up new avenues for antibiotic development, challenging the conventional wisdom that effective antibiotics must target essential bacterial processes. Prof. Kaiser emphasised the broader implications of their findings: “In further research work, we now need to clarify precisely how callyaerins interact with Rv2113 and how this interaction disrupts various cellular processes in such a way that M. tuberculosis can no longer grow. However, we have been able to show that non-essential proteins can also represent valuable target structures for the development of novel antibiotics.”
The road ahead
While the discovery of callyaerins and their unique mechanism of action is promising, significant work remains before these compounds can be translated into clinical treatments. Further research is needed to elucidate the precise interactions between callyaerins and Rv2113, as well as to optimise the compounds for potential therapeutic use.
Nevertheless, this breakthrough offers new hope in the ongoing battle against tuberculosis, particularly in the face of rising antibiotic resistance. By targeting non-essential bacterial proteins, researchers may be able to develop new classes of antibiotics that are less susceptible to resistance development, potentially revolutionising the treatment of TB and other infectious diseases.
As the global medical community continues to grapple with the challenges posed by antibiotic-resistant pathogens, innovative approaches like those demonstrated by the callyaerin research will be crucial in developing the next generation of antimicrobial therapies. The success of this study not only highlights the potential of marine-derived compounds in drug discovery but also underscores the importance of exploring unconventional targets in the fight against infectious diseases.
Reference
- Podlesainski, D., Adeniyi, E. T., Gröner, Y., et. al. (2024). The anti-tubercular callyaerins target the Mycobacterium tuberculosis-specific non-essential membrane protein Rv2113. Cell Chemical Biology, 31. https://doi.org/10.1016/j.chembiol.2024.06.002

