Innovative homebased clinical polysomnography solution set to enter market later this year
International Hospital speaks to Ruben de Francisco, CEO of Onera Health, an Eindhoven-based startup, about their new, innovative, clinicalgrade patch-based polysomnography system that a patient can use at home to capture data for diagnosing sleeprelated disorders and monitoring sleep therapy.
International Hospital: Your startup company, Onera Health has developed clinical-grade, disposable patches that a patient can use at home to diagnose sleep-related disorders and monitor sleep-related therapy. We’ll come back to this, but first let’s look at the company. What was the seed idea that led to you starting up Onera, and when did the idea originate?
Ruben de Francisco: Over the last two decades, the field of sleep health and medicine shifted increasingly into the focus of society and, consequently, of R&D. Onera was established on the foundation of a long research period at imec, an internationally renowned research & development organization active in the fields of nanoelectronics and digital technologies. The four Onera founders were intrigued by the possibilities and necessity to revolutionize remote diagnostics and monitoring. Moreover, with our diverse background, we were eager to bring sleep medicine’s outdated technology into the future and help people to get diagnosed.
IH: Can you outline some of the initial processes / research that led to the establishment of Onera?
RdF: Onera started within imec and soon became an independent spin-off company that to this day works in partnership with imec during the development of various Onera products. From the very beginning, Onera worked close to the field, starting from comprehensive market validation to determine the needs of clinics, hospitals, and key opinion leaders. The business validation was followed by an extensive research phase with clinical studies to understand further what is needed from Onera’s diagnostic and monitoring solutions in the future.
IH: When and where did you establish the company?
RdF: Onera was establish in April 2017 in Eindhoven, the Netherlands.
IH: Seed funding is a key issue for startup initiatives. Can you explain how you raised seed funding and what the initial funding was used for?
RdF: The passion for Onera was tangible from the getgo, the pitches were many and strong, and early on, we had great supporters who believed in our vision and promising business proposal. Furthermore, we benefited from a great Dutch community in the Brainport region and beyond and were rewarded with diverse accelerator programmes, like HighTechXL. The initial funding was used to literally launch the company: hiring a talented and passionate team, office space, etc. And Onera is always looking for new bright minds, even if that means risking outgrowing our office space a third time.
IH: You have received funding from the European Horizon 2020 initiative. What other funding and/or investors have come on board?
RdF: Yes, Onera was rewarded with the prestigious European Innovation Council (EIC) Accelerator (SME Instrument) grant as part of the European Horizon 2020 programme to develop further breakthrough innovations in medical-grade remote care. Furthermore, we also received an OP-Zuid R&D Grant in partnership with TU/e and Kempenhaeghe, the Eurostar R&D grant in association with Verhaert and an additional European Institute of Innovation & Technology grant in cooperation with the University of Trento and Management Innovation for a joint project.
From seed investment by imec, imec.xpand and the Brabantse Ontwikkelings Maatschapij (BOM), to the Series A round led by Jazz Pharmaceuticals and imec.xpand, which added 15th Rock as a new investor, to the most recent series B funding led by Innovation Industries and Invest-NL, we consider ourselves incredibly fortunate to have seven strong supporters backing Onera beyond financial means.
IH: How have you managed expansion of the company? What were some of the main challenges you faced along the way and how did you resolve these?
RdF: Key moments were closing the Series A and B funding, supported by the grants, which allowed us to develop a broader portfolio of Onera products. The biggest challenge is finding the right talents to support our existing team during a pandemic and the shortages along the global supply chains that all companies in the technology sector currently face. Onera’s core technology includes semiconductor technology, such us the recently launched Onera Biomedical-Lab-on-Chip, which helps us to address some of the supply chain risks.
IH: Let’s look at your key products. Can you tell us about the disposable patch for sleep diagnostics?
RdF: We received the CE marking for commercialisation in Europe for our Onera STS I, a patch-based polysomnography (PSG) system for sleep studies, in June 2021. This will enable us to launch our end-to-end solution for clinical-grade sleep studies, Onera PSG-as-a-Service, which we aim to introduce later this year. In this important phase, it is crucial to us to continue with how we started: working closely with the field and meeting the needs of sleep clinics, doctors and their patients. We introduced the technology already to a group of sleep experts who are currently using the solution in their practices before launching it broadly later this year.
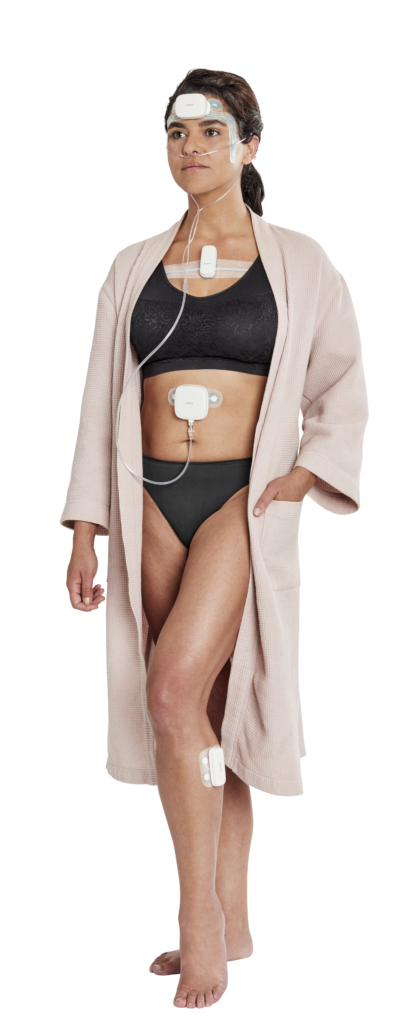
IH: If we look at the patch itself. How many patches will a patient need to use? Where should they be placed on the body and what parameters do they measure?
RdF: Onera STS I consists of four sensors for one single night study, they are applied on the forehead, upper chest, abdominal area, and lower leg. The signals recorded include the electroence-phalogram (EEG), which reflects changes in brain activity, electromyography (EMG) to measure jaw muscle activity and electro-oculography (EOG) to register eye movements. Additional physiological data includes respiratory measurements, electrocardiogram (ECG), electromyography leg, sound pressure (to detect snoring), body motion and body position. Depending on their hypotheses, a clinician ordering a sleep study may require the full STS I signal set or a subset to record and diagnose various sleep disorders.
IH: I presume the data will be transferred by Wifi to an app and that this data will then be used by a clinician to provide a treatment plan or is Onera planning to do this in-house?
RdF: The data is stored on the Onera STS I pods during the night. Depending on the distribution model, they are either returned to the clinic or sent to Onera. The data is then uploaded to the webbased Onera Digital Health Platform, analysed, and a report for the physician is created, who will interpret it to come to a diagnosis for the respective patient. This simplified set-up of a sleep study will allow sleep doctors to focus on those parts of the diagnostic journey that create the highest value and also spending more time for interaction with the patient and evaluation of potential treatments.
IH: What types of sleep disorders will the patches be able to diagnose with these parameters?
RdF: A clinician can use Onera STS to diagnose a broad range of sleep disorders, similarly to what they would be able to diagnose with a traditional PSG system in a sleep study. Onera STS I’s innovative sensors use non-invasive technology that records multiple physiological parameters related to cardiovascular, respiratory and brain functions and stores comprehensive polysomnography data. Onera’s solutions bring the quality of a clinical-grade PSG diagnosis directly to the patients’ bed – whether in the hospital, at the sleep lab, or even at home.
Depending on their hypotheses, a sleep doctor can have a closer look at the multitude of physiological parameters related to cardiovascular, respiratory and brain functions recorded by Onera STS I sensors. These parameters or a combination of those are a tell-tell sign for various sleep disorders, from Obstructive Sleep Apnoea and Snoring to Insomnia and Narcolepsy.
IH: What sets this product apart from similar competitive devices?
RdF: Onera’s ground-breaking PSG-as-a-Service solution using its patch-based recording technology is the first no-wire solution that can be used anywhere. The simplicity and intuitive design of the Onera STS I offers new opportunities for sleep clinics to expand their sleep programmes and for hospitals that have limited or no access to a sleep clinic to implement sleep diagnostic testing within their institution. Whether a sleep study requires the full STS I signal set or a subset; the recorded data allows to diagnose various sleep disorders leaving previous limitations of Home Sleep Tests in the past.
IH: When do you expect the product to be commercialised?
RdF: After the clinical trials as well as successful pilot projects, we are aiming to launch Onera PSG-as-a-Service with the STS I in the second half of this year, 2022.
IH: Do you have other products in the pipeline? Can you tell us a bit about these?
RdF: Onera is constantly working towards broadening our diagnostic and monitoring solutions, for sleep medicine but also beyond. As part of our portfolio, we recently launched our Onera Biomedical-Lab-on-Chip, an ultra-low-power biosignal sensor hub. The biomedical sensor system-on-chip acquires and processes multiple biosignals and is used in our own solutions. Still, it is designed as a stand-alone product for a broad range of wearable health applications and devices, offering many solutions and opportunities for innovation in the medical, wellness and fitness space.
IH: How can parties who are interested to invest in your company go about doing this?
RdF: We are always interested and open to partnerships, be it as a supporter or through collaborations. The easiest way is to go through our contact form on our website: www.onerahealth.com/contact.
IH: John Baekelmans, Managing Director and Vice President at imec, is quoted as saying ‘Eindhoven is a great incubator for startups’. Can you tell us a bit more about this?
RdF: Eindhoven is a high-tech hotspot known for close cooperation between industry, educational and knowledge institutes, and public authorities. The shared knowledge and the created networks are invaluable for innovations; paired with renowned accelerator programmes and strong local investors, it is an attractive place for startups.
IH: What are some of the key things you have learnt in this startup process that you can pass on to others who are about to begin this journey?
RdF: It might be challenging, especially when you are passionate about your product (or product to be), but patience is vital. Involve your stakeholders from day one, keep the customers’ needs in mind, do not make any assumptions, listen, and verify it throughout its lifecycle.