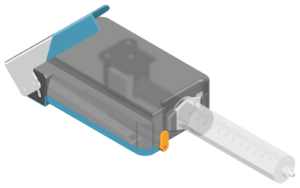
IHS announces CE Mark for Insignis Syringe Infusion System
Innovative Health Sciences has received ISO 13485 quality management system and CE Mark certification for the Insignis Syringe Infusion System.
The Insignis Syringe Infusion System is the first combination of intravenous and subcutaneous non-electric infusion pump created for use with a selectable rate flow controller, the IV Controller and the OneSett.
The Insignis System for intravenous administration answers the need for a portable, non-electric, versatile, cost-effective, and intuitive infusion pump to accurately deliver medications from KVO up to 250 ml/hr with a direct reading selectable rate flow controller.
For subcutaneous applications, the OneSett consists of a selectable rate flow controller attached directly to a subcutaneous needle set (1-4 leg configuration offering), which enables the user to select the flow rate (from 10 ml – 60 ml) with the turn of a dial. The flow rate indicated on the dial is delivered to each needle site – no calculation required.
The IHS infusion system addresses nearly every infusion therapy need and can support intermittent, ‘pause’ dosing, and is ideal for lengthy low-volume hospital infusions.
“The IHS system represents a paradigm shift in simplicity, excellence, cost effectiveness, usability and patient-centred design,” said Andrew Sealfon, IHS founder, Chairman, and CTO.